Axillary Hyperhidrosis Treatment Market Accelerates Across APAC, Europe, USA & Saudi Arabia Through 2035
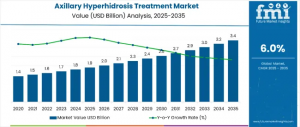
Global axillary hyperhidrosis treatment market to grow from USD 1.9B (2025) to USD 3.4B (2035), driven by awareness, innovation, and personalized care models.
AK, UNITED STATES, November 7, 2025 /EINPresswire.com/ -- The global axillary hyperhidrosis treatment market is set for notable expansion as awareness of excessive underarm sweating and its psychological, social, and professional implications continues to rise. The market, valued at USD 1.9 billion in 2025, is projected to reach USD 3.4 billion by 2035, reflecting a 6% CAGR. Increasing demand for long-lasting, non-invasive solutions and personalized treatment pathways is significantly influencing market dynamics across the United States, Europe, Asia-Pacific (APAC), and Saudi Arabia.
Rising Awareness and Shifting Treatment Preferences
Hyperhidrosis affects millions globally, often leading to discomfort, embarrassment, and lowered self-esteem. While traditional antiperspirants and medicated wipes remain common, consumers are increasingly pursuing solutions with extended effectiveness. Drug therapies, including topical anticholinergics and botulinum toxin injections, currently hold an estimated 65% share of the treatment segment, attributed to their adaptability and clinical effectiveness.
North America continues to command the largest share of the market due to early access to FDA-approved solutions, broad insurance coverage, and a well-established dermatology care network. Meanwhile, South Asia-Pacific, led by India, is poised for the fastest growth, projected at 10.1% CAGR through 2035, driven by rising middle-class affordability and expanding private healthcare clinics.
Innovation Fuels Market Evolution
Recent product launches and clinical advancements are driving a shift toward less invasive, longer-lasting solutions.
• Sofpironium bromide gel and the Brella SweatControl Patch have demonstrated effective outcomes for patients unresponsive to standard antiperspirants.
• Microwave thermolysis devices and next-generation botulinum toxin formulations such as DaxibotulinumtoxinA (Daxi) are improving treatment duration, patient adherence, and comfort levels.
Additionally, telemedicine and online pharmacy platforms are expanding access to diagnosis and home-based treatment guidance, especially in underserved regions.
Regional Market Performance
• United States: Expected growth at 3.2% CAGR, supported by insurance expansion and strong provider uptake of microwave thermolysis and Botox-related interventions.
• Germany (Western Europe): Maintains robust adoption due to a structured insurance system and high dermatology clinic density, growing at 3.8% CAGR.
• India (South Asia-Pacific): Highest regional growth trajectory (10.1% CAGR) driven by awareness campaigns, affordability improvements, and lifestyle factors.
• Saudi Arabia and GCC: Growth supported by private-sector clinic investments and aesthetic medicine adoption trends.
Distribution Channel Outlook
Retail pharmacies represent approximately 35% of distribution volume in 2025, driven by easy accessibility and consumer preference for OTC-based self-care. However, online sales channels are rapidly gaining traction, especially for medicated wipes, clinical-strength roll-ons, and refill-prescription support.
Market Challenges
Despite promising expansion, several barriers still affect treatment adoption:
• Effects of botulinum injections typically last only 3–6 months, requiring repeated visits.
• High out-of-pocket costs in regions with limited insurance coverage.
• Anticholinergic drug side effects, including dry mouth and blurred vision, affecting long-term compliance.
Growth Opportunities Ahead
The future of market development lies in:
• Combination therapy models, integrating topical agents with procedure-based interventions.
• Insurance policy expansion across emerging and developed markets.
• Personalized treatment, driven by sweat-mapping diagnostics and patient lifestyle profiling.
Competitive Landscape
The market is moderately consolidated, with Tier 1 companies holding approximately 54.4% share, including Allergan (AbbVie), GlaxoSmithKline, Duradry, and Dove. Tier 2 and Tier 3 players strengthen market adaptability through cost-effective innovations, localized formulations, and targeted digital patient outreach.
Recent strategic developments include:
• June 2024: Botanix Pharmaceuticals secured FDA approval for Sofdra, a topical gel for primary axillary hyperhidrosis.
• January 2025: Dermata Therapeutics and Revance Therapeutics launched a joint clinical evaluation of topical and injectable combination therapy regimens.
Latest Therapy Area Reports:-
Kids Splint Market
https://www.futuremarketinsights.com/reports/kids-splint-market
Ophthalmic Eye Drop Market
https://www.futuremarketinsights.com/reports/ophthalmic-eye-drops-market
GLP-1 Receptor Agonist Market
https://www.futuremarketinsights.com/reports/glp-1-receptor-agonist-market
Why Choose FMI Empowering Decisions that Drive Real-World Outcomes:- https://www.futuremarketinsights.com/why-fmi
About Future Market Insights (FMI)
Future Market Insights, Inc. (ESOMAR certified, recipient of the Stevie Award, and a member of the Greater New York Chamber of Commerce) offers profound insights into the driving factors that are boosting demand in the market. FMI stands as the leading global provider of market intelligence, advisory services, consulting, and events for the Packaging, Food and Beverage, Consumer Technology, Healthcare, Industrial, and Chemicals markets. With a vast team of over 400 analysts worldwide, FMI provides global, regional, and local expertise on diverse domains and industry trends across more than 110 countries.
Contact Us:
Future Market Insights Inc.
Christiana Corporate, 200 Continental Drive,
Suite 401, Newark, Delaware – 19713, USA
T: +1-347-918-3531
For Sales Enquiries: sales@futuremarketinsights.com
Website: https://www.futuremarketinsights.com
LinkedIn| Twitter| Blogs | YouTube
Sudip Saha
Future Market Insights Inc.
+1 347-918-3531
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.