United States Oncology Biosimilars Market is expected to reach US$ 30.83 Billion by 2033 | DataM Intelligence
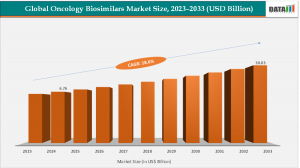
The Global Oncology Biosimilars Market is expected to reach at a CAGR of 18.6% during the forecast period 2025-2033.
The Oncology Biosimilars Market is expanding rapidly, driven by rising cancer prevalence, patent expiries of biologics, and growing demand for affordable cancer therapies.”
AUSTIN, TX, UNITED STATES, November 4, 2025 /EINPresswire.com/ -- Overview of the Market:— DataM Intelligence
The Oncology Biosimilars Market has emerged as one of the most transformative segments in the pharmaceutical industry, reshaping cancer treatment accessibility and affordability worldwide. As cancer incidence rates continue to surge globally, biosimilars biological products highly similar to already approved biologic drugs are bridging the gap between innovation and affordability in oncology therapeutics. According to DataM Intelligence, The Global Oncology Biosimilars Market was valued at US$ 6.76 billion in 2024 and is projected to reach US$ 30.83 billion by 2033, growing at a CAGR of 18.6% during the forecast period (2025–2033). This robust growth is attributed to increasing patent expirations of blockbuster biologics, the rising prevalence of cancer, and growing awareness about cost-effective biologic therapies.
To Download Sample Report Here: https://www.datamintelligence.com/download-sample/oncology-biosimilars-market
The Increasing adoption of targeted therapies and monoclonal antibodies in cancer treatment has created a substantial demand for biosimilars. Among the product segments, monoclonal antibody biosimilars dominate the market due to their wide application in treating cancers such as breast, colorectal, and lung cancer. Regionally, Europe holds the largest share of the market owing to supportive regulatory pathways, early adoption, and favorable reimbursement policies. However, North America and Asia-Pacific are anticipated to witness the fastest growth, driven by ongoing regulatory reforms and rising oncology drug costs that are pushing healthcare systems toward biosimilar alternatives.
Key Highlights from the Report:
Growing adoption of oncology biosimilars due to increasing cancer prevalence worldwide.
Patent expirations of key biologic drugs like Herceptin, Avastin, and Rituxan drive biosimilar entry.
Europe remains the leading market due to strong biosimilar uptake and regulatory support.
Asia-Pacific is expected to show the fastest growth owing to expanding healthcare access and investments.
Rising demand for cost-effective cancer therapies boosts the market across emerging economies.
Increasing strategic collaborations and R&D investments among biopharma companies accelerate innovation.
Market Segmentation:
The Oncology Biosimilars Market is segmented based on product type, cancer type, and end-user.
By product type, monoclonal antibody biosimilars lead the market, driven by their widespread use in oncology treatment regimens. Biosimilars of Trastuzumab (Herceptin), Bevacizumab (Avastin), and Rituximab (Rituxan) are among the most prescribed due to their proven therapeutic efficacy and affordability. Other biosimilar categories include erythropoietin, granulocyte colony-stimulating factor (G-CSF), and recombinant human insulin, which also play crucial roles in managing cancer-related conditions.
By cancer type, breast cancer dominates the biosimilars landscape as it represents one of the largest patient populations treated with biologics. Trastuzumab biosimilars, for instance, have significantly reduced treatment costs and improved patient access globally. Other major segments include lung cancer, colorectal cancer, and blood cancers, where biosimilars of monoclonal antibodies are gaining strong traction.
In terms of end-users, hospitals constitute the largest segment due to the increasing use of biosimilars in oncology departments and specialized cancer centers. Oncology clinics and research institutions also represent key users, especially in early-stage trials and cost-sensitive therapeutic settings.
Get Customization in the report as per your requirements: https://www.datamintelligence.com/customize/oncology-biosimilars-market
Regional Insights:
Europe remains the most mature and dominant region in the oncology biosimilars market. The region’s well-defined regulatory framework, led by the European Medicines Agency (EMA), has enabled the early approval and adoption of biosimilars. Countries such as Germany, the UK, and France have seen strong integration of biosimilars into healthcare systems, supported by reimbursement mechanisms that favor cost savings.
North America, particularly the United States, is witnessing accelerated biosimilar adoption following the establishment of the Biologics Price Competition and Innovation Act (BPCIA). As more patents expire for major biologics, the U.S. market is expected to experience substantial price competition and wider biosimilar usage in oncology care.
In the Asia-Pacific region, countries like India, China, and South Korea are becoming global hubs for biosimilar development and manufacturing. Affordable pricing strategies, growing domestic biopharma capabilities, and government incentives are driving growth. Meanwhile, Latin America and the Middle East & Africa are in the early stages of biosimilar penetration but show significant potential due to the rising cancer burden and limited healthcare budgets.
Market Dynamics:
Market Drivers
The primary driver of the oncology biosimilars market is the increasing global cancer incidence, coupled with the high cost of reference biologics. Biosimilars offer comparable efficacy at significantly lower costs, making them a crucial component in reducing the economic burden on healthcare systems. Patent expirations of leading biologic drugs such as Herceptin, Avastin, and Rituxan have opened the door for multiple biosimilar entrants. Additionally, favorable regulatory frameworks, technological advancements in biologic production, and growing physician acceptance are fueling adoption.
Market Restraints
Despite the promising outlook, the market faces several challenges. The complex manufacturing process and stringent regulatory requirements often result in high development costs and extended approval timelines. Moreover, physician skepticism and limited awareness among patients in developing regions hinder widespread acceptance. The patent litigation and exclusivity strategies adopted by original biologic manufacturers further delay biosimilar launches, restricting market access.
Market Opportunities
The future holds immense potential for oncology biosimilars. Emerging markets offer vast growth opportunities due to increasing healthcare infrastructure investments and government-driven cost-control initiatives. Furthermore, technological advancements in bioprocessing and strategic alliances between global pharmaceutical giants and regional biopharma firms are expected to enhance biosimilar development pipelines. Personalized medicine trends and the growing emphasis on value-based care models also create new pathways for biosimilar integration into cancer treatment.
Frequently Asked Questions (FAQs):
How Big is the Oncology Biosimilars Market?
What is the Projected Growth Rate of the Oncology Biosimilars Market from 2025 to 2033?
Who are the Key Players Operating in the Global Oncology Biosimilars Market?
Which Region is Expected to Lead the Market Through the Forecast Period?
What are the Major Factors Driving the Demand for Oncology Biosimilars Worldwide?
Company Insights:
Key players operating in the Oncology Biosimilars Market include:
Celltrion USA, Inc.
Amgen Inc.
Pfizer Inc.
Biocon Biologics Limited
Teva Pharmaceuticals USA, Inc.
Organon group of companies
Accord BioPharma
Sandoz Inc.
Recent Developments:
United States:
In November 2025, the oncology biosimilars market continued rapid expansion driven by cost-effective alternatives to biologic therapies, with advancements in bioprocessing and clinical development enabling increased adoption and improved patient care.
In October 2025, ongoing steep price discounts and payer/provider adoption of biosimilars targeting blockbuster biologics have facilitated broader market penetration and cost savings in oncology treatments in the US.
Japan:
In November 2025, Celltrion's biosimilar therapy Herzuma (trastuzumab) for breast and gastric cancer held a 74% market share in Japan, maintaining market leadership and growing against competitors.
In February 2025, Biocon Biologics collaborated with Janssen to launch a biosimilar targeting autoimmune diseases, including Japan in its commercial territories, signaling increasing partnerships and expansion in the oncology biosimilars market there.
Unlimited Insights. One Subscription: https://www.datamintelligence.com/reports-subscription
Conclusion:
The Oncology Biosimilars Market represents a paradigm shift in global cancer therapy, offering patients access to advanced biologics at affordable costs. As healthcare systems increasingly focus on cost containment and equitable access, biosimilars are expected to play a pivotal role in shaping the future of oncology care. Supported by a strong regulatory environment, ongoing innovation, and expanding healthcare coverage, the market is poised for substantial growth through 2031. With key players investing in research, partnerships, and education initiatives, oncology biosimilars will continue to redefine therapeutic accessibility and sustainability in cancer treatment worldwide.
Related Reports:
Breast Cancer Therapeutics Market
Targeted Therapy Market
Sai Kiran
DataM Intelligence 4Market Research
+1 877-441-4866
Sai.k@datamintelligence.com
Visit us on social media:
LinkedIn
X
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.