Chronic Gout Market to Witness Strong Growth by 2034 Owing to the Expected Launch of Therapies including mTOR Inhibitors and URAT1 Inhibitors | DelveInsight
The chronic gout market is expected to maintain a stable growth over the forecast period (2025–2034). This growth is fueled by increasing prevalence due to aging populations and cardiometabolic comorbidities, enhanced diagnosis and awareness, a robust R&D pipeline, including xanthine oxidase inhibitors, uricosurics, recombinant uricases, and targeted anti-inflammatory therapies, expanded adoption of advanced diagnostic tools, higher healthcare expenditure, and the unmet needs in patients with refractory disease.
New York, USA, Sept. 02, 2025 (GLOBE NEWSWIRE) -- Chronic Gout Market to Witness Strong Growth by 2034 Owing to the Expected Launch of Therapies including mTOR Inhibitors and URAT1 Inhibitors | DelveInsight
The chronic gout market is expected to maintain a stable growth over the forecast period (2025–2034). This growth is fueled by increasing prevalence due to aging populations and cardiometabolic comorbidities, enhanced diagnosis and awareness, a robust R&D pipeline, including xanthine oxidase inhibitors, uricosurics, recombinant uricases, and targeted anti-inflammatory therapies, expanded adoption of advanced diagnostic tools, higher healthcare expenditure, and the unmet needs in patients with refractory disease.
DelveInsight’s Chronic Gout Market Insights report includes a comprehensive understanding of current treatment practices, emerging chronic gout drugs, market share of individual therapies, and current and forecasted chronic gout market size from 2020 to 2034, segmented into leading markets (the US, EU4, UK, and Japan).
Chronic Gout Market Summary
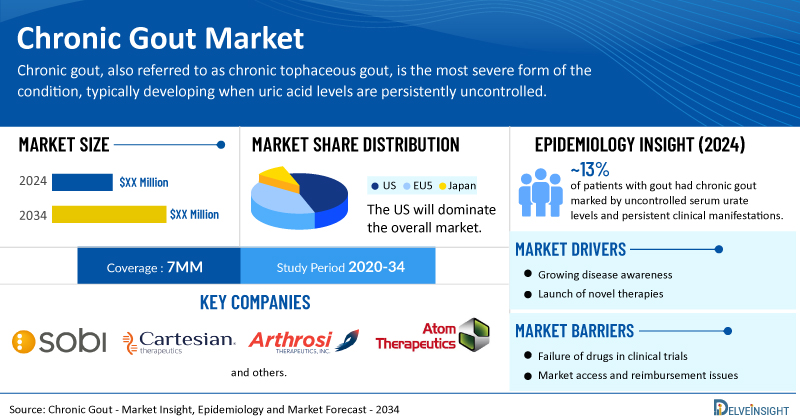
- The total chronic gout treatment market size is expected to grow positively by 2034 in the leading markets (the US, EU4, UK, and Japan).
- The United States accounts for the largest market size of chronic gout, in comparison to EU4 (Germany, Italy, France, and Spain), the UK, and Japan.
- As per the secondary analysis, approximately 13% of patients with gout had chronic gout marked by uncontrolled serum urate levels and persistent clinical manifestations.
- Key chronic gout companies, including Sobi, Cartesian Therapeutics, Arthrosi Therapeutics, Atom Therapeutics, and others, are actively working on innovative chronic gout drugs.
- Some of the key chronic gout therapies in clinical trials include NASP (formerly SEL-212), Pozdeutinurad (AR882), Lingdolinurad (ABP-671), and others. These novel chronic gout therapies are anticipated to enter the chronic gout market in the forecast period and are expected to change the market.
Discover which chronic gout medications are expected to grab the market share @ Chronic Gout Market Report
Key Factors Driving the Growth of the Chronic Gout Market
Rising Chronic Gout Prevalence Linked to Lifestyle Changes
The global incidence of gout has been increasing, with over 53 million cases reported in 2024. The prevalence of gout among US adults is 3.9%, with a higher burden in men at 5.2% and in women at 2.7%. This surge is attributed to lifestyle factors such as poor diet, excessive alcohol consumption, and obesity, which elevate uric acid levels and contribute to the development of chronic gout.
Emergence of New Drug Classes in Chronic Gout
The chronic gout market is expected to surge, primarily driven by the development of mTOR inhibitors, such as NASP (Sobi/Cartesian Therapeutics), and URAT1 inhibitors, including Pozdeutinurad (Arthrosi Therapeutics) and Lingdolinurad (Atom Therapeutics).
Launch of Emerging Chronic Gout Therapies in Forecast Period (2025-2034)
The anticipated launch of emerging chronic gout therapies such as NASP (Sobi/Cartesian Therapeutics), pozdeutinurad (Arthrosi Therapeutics), lingdolinurad (Atom Therapeutics), and others during the forecast period (2025-2034) will propel the chronic gout market.
Increasing Awareness and Early Diagnosis
There is a growing emphasis on patient education and self-management programs, which focus on lifestyle modifications, dietary changes, and the importance of adhering to medication. These initiatives are gaining traction and are expected to contribute to the market's growth by promoting early diagnosis and preventive management of chronic gout.
Chronic Gout Market Analysis
The current treatment options for chronic gout, including allopurinol, febuxostat, and probenecid, are available as generics, providing widely accessible and cost-effective options for long-term urate-lowering therapy. In contrast, KRYSTEXXA (pegloticase) is a branded, FDA-approved biologic indicated explicitly for adults with chronic gout that is refractory to conventional treatments. KRYSTEXXA is a PEGylated, uric acid–specific enzyme administered intravenously, which works by converting uric acid into allantoin, aa more soluble compound that is easily excreted, effectively reducing serum urate levels in patients whose gout remains uncontrolled despite standard therapy. Supporting its position in the rare disease space, Amgen acquired Horizon Therapeutics in October 2023 in a deal valued at approximately USD 27.8 billion, thereby expanding its portfolio. KRYSTEXXA itself has achieved key regulatory milestones, including FDA approval for gout treatment in September 2010 and, more recently, an expanded label in July 2022 allowing co-administration with methotrexate for patients with uncontrolled gout.
Learn more about the chronic gout treatment options @ Chronic Gout Treatment Guidelines
Chronic Gout Competitive Landscape
Companies such as Sobi, Cartesian Therapeutics, Arthrosi Therapeutics, Atom Therapeutics, and others are actively working in the chronic gout clinical trial landscape with their lead assets. A few of the emerging therapies, such as NASP, pozdeutinurad, and lingdolinurad, among others, are currently in chronic gout clinical trials to improve the treatment landscape.
NASP (nanoencapsulated sirolimus plus pegadricase) by Sobi/Cartesian Therapeutics is an innovative, once-monthly investigational therapy for the treatment of chronic refractory gout. It is designed to lower serum urate levels and reduce tissue urate deposits, which, if left untreated, can cause gout flares and joint deformities. In July 2024, Sobi initiated a rolling Biologics License Application (BLA) submission to the US FDA for NASP (formerly SEL-212), supported by pivotal data from the DISSOLVE I and II trials, with a regulatory decision expected in 2026. This milestone follows the FDA's granting of Fast Track designation to NASP in March 2024, underscoring the need for new treatments for chronic, refractory gout. Since July 2020, Sobi has held the rights to develop, register, and commercialize NASP outside China under a licensing agreement with Cartesian Therapeutics, which includes up to approximately USD 585 million in milestone payments and double-digit royalties on future net sales.
Pozdeutinurad (AR882) is a highly potent, selective next-generation urate transporter 1 (URAT1) inhibitor under development for gout treatment. It aims to lower serum urate, prevent flares, and resolve tophi. AR882 is currently in Phase III for chronic gout management and Phase II for refractory tophaceous gout. In Phase II trials, AR882 demonstrated superior efficacy over standard therapies, with the 75 mg dose reducing serum urate to <4 mg/dL in 88% of patients, compared to none with allopurinol or febuxostat. It also achieved complete tophi resolution in a Phase IIb study while maintaining a favorable safety profile.
In June 2025, Arthrosi Therapeutics presented two studies at the EULAR Congress in Barcelona, emphasizing AR882’s potential to reduce serum urate, prevent flares, and dissolve tophi in patients with gout and tophaceous gout. In March 2025, the company reported two major milestones in its Phase III program: early completion of enrollment in the pivotal global REDUCE 2 trial and dosing of the first patient in the replicate REDUCE 1 trial, both evaluating AR882’s efficacy in lowering serum urate and resolving tophi. By December 2024, over 50% of patients had been enrolled in the REDUCE 2 trial. Additionally, AR882 received FDA Fast Track designation in August 2024 for the potential treatment of clinically visible tophi.
ABP-671 is a once-daily oral URAT1 inhibitor that reduces uric acid reabsorption in the kidneys, increasing urinary excretion and lowering serum uric acid (sUA) levels. Long-term use can help dissolve tophi, prevent gout flares, and mitigate hyperuricemia-related complications, including cardiovascular, renal, and metabolic disorders. Clinical guidelines recommend maintaining serum uric acid (sUA) levels below 6 mg/dL to prevent new crystal formation and below 5 mg/dL to accelerate tophus resolution. In a Phase IIa trial, ABP-671 achieved these targets in 93% (<6 mg/dL) and 77% (<5 mg/dL) of patients, supporting its progression to global Phase III trials and commercialization by Atom Therapeutics.
In May 2025, Atom Therapeutics participated in the Gout Education Society’s Gout Awareness Day to educate the public on gout risk factors and treatment options. Its lead candidate, lingdolinurad (ABP-671), is currently in Phase IIb/III trials for chronic gout and hyperuricemia. In November 2024, Atom presented positive Phase I data at the ACR Convergence, showing ABP-671 was safe, well-tolerated, and effectively reduced serum uric acid in participants with mild to moderate chronic kidney disease. The therapy continues to advance in global Phase IIb/III trials.
The anticipated launch of these emerging chronic gout therapies are poised to transform the chronic gout market landscape in the coming years. As these cutting-edge chronic gout therapies continue to mature and gain regulatory approval, they are expected to reshape the chronic gout market landscape, offering new standards of care and unlocking opportunities for medical innovation and economic growth.
To know more about new treatment for chronic gout, visit @ Chronic Gout Management
Recent Developments in the Chronic Gout Market
- In June 2025, Arthrosi Therapeutics presented two studies at the European Alliance of Associations for Rheumatology (EULAR) Congress in Barcelona, highlighting the potential of its next-generation URAT1 inhibitor for reducing serum urate levels, flares, and dissolving tophi in patients with gout and tophaceous gout.
- In May 2025, Atom Therapeutics joined the Gout Education Society’s Gout Awareness Day to raise public awareness about the risk factors and treatment options for gout.
- In March 2025, Arthrosi Therapeutics announced two key milestones in its Phase III program for pozdeutinurad (AR882).
Chronic Gout Epidemiology Segmentation
The chronic gout market forecast is derived using patient-based forecasts and analysis. The report offers in-depth analysis of the historical and current chronic gout patient pool and forecasted trends for the leading markets (the US, EU4, UK, and Japan). It helps recognize the causes of current and forecasted patient trends by exploring numerous studies and views of key opinion leaders.
The chronic gout market report proffers epidemiological analysis for the study period 2020–2034 in the leading markets (the US, EU4, UK, and Japan) segmented into:
- Total Diagnosed Prevalent Cases of Gout
- Type-specific Diagnosed Prevalent Cases of Gout
- Total Diagnosed Prevalent Cases of Chronic Gout
- Treated Cases of Chronic Gout
Download the report to understand which factors are driving chronic gout epidemiology trends @ Chronic Gout Treatment Algorithm
| Chronic Gout Market Report Metrics | Details |
| Study Period | 2020–2034 |
| Chronic Gout Market Report Coverage | 7MM [The United States, the EU-4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan] |
| Chronic Gout Epidemiology Segmentation | Total Diagnosed Prevalent Cases of Gout, Type-specific Diagnosed Prevalent Cases of Gout, Total Diagnosed Prevalent Cases of Chronic Gout, and Treated Cases of Chronic Gout |
| Emerging Classes in the Chronic Gout Market | mTOR Inhibitors and URAT1 Inhibitors |
| Key Chronic Gout Companies | Sobi, Cartesian Therapeutics, Arthrosi Therapeutics, Atom Therapeutics, Amgen, and others |
| Key Chronic Gout Therapies | NASP (formerly SEL-212), Pozdeutinurad (AR882), Lingdolinurad (ABP-671), KRYSTEXXA, and others |
Scope of the Chronic Gout Market Report
- Chronic Gout Therapeutic Assessment: Chronic Gout current marketed and emerging therapies
- Chronic Gout Market Dynamics: Conjoint Analysis of Emerging Chronic Gout Drugs
- Competitive Intelligence Analysis: SWOT analysis and Market entry strategies
- Chronic Gout Market Unmet Needs, KOL’s views, Analyst’s views, Chronic Gout Market Access and Reimbursement
Discover more about chronic gout drugs in development @ Chronic Gout Clinical Trials
Table of Contents
| 1 | Chronic Gout Market Key Insights |
| 2 | Chronic Gout Market Report Introduction |
| 3 | Chronic Gout Market Overview at a Glance |
| 3.1. | Chronic Gout Market Share (%) Distribution by Therapies in 2024 |
| 3.2. | Chronic Gout Market Share (%) Distribution by Therapies in 2034 |
| 4 | Chronic Gout Epidemiology and Market Methodology |
| 5 | Executive Summary |
| 6 | Key Events |
| 7 | Disease Background and Overview |
| 7.1. | Introduction |
| 7.2 | Chronic Gout Types |
| 7.3. | Chronic Gout Causes |
| 7.4. | Chronic Gout Pathophysiology |
| 7.5. | Chronic Gout Symptoms |
| 7.6. | Chronic Gout Risk Factor |
| 7.7. | Chronic Gout Diagnosis |
| 7.8. | Chronic Gout Treatment and Management |
| 8 | Chronic Gout Epidemiology and Patient Population |
| 8.1 | Key Findings |
| 8.2 | Assumptions and Rationale: The 7MM |
| 8.3 | Total Diagnosed Prevalent Cases of Chronic Gout in the 7MM |
| 8.4 | The US |
| 8.4.1 | Total Diagnosed Prevalent Cases Of Gout |
| 8.4.2 | Type-specific Diagnosed Prevalent Cases of Gout |
| 8.4.3 | Total Diagnosed Prevalent Cases Of Chronic Gout |
| 8.4.4 | Treated Cases of Chronic Gout |
| 8.5 | EU4 and the UK |
| 8.6 | Japan |
| 9 | Chronic Gout Patient Journey |
| 10 | Marketed Chronic Gout Therapies |
| 10.1 | KRYSTEXXA (pegloticase): Amgen |
| 10.1.1 | Drug Description |
| 10.1.2 | Regulatory Milestones |
| 10.1.3 | Other Development Activities |
| 10.1.4 | Clinical Trials Information |
| 10.1.5 | Safety and Efficacy |
| To be continued in the report…. | |
| 11 | Emerging Chronic Gout Therapies |
| 11.1 | Key Cross Competition |
| 11.2 | NASP (formerly SEL-212): Swedish Orphan Biovitrum (Sobi)/Cartesian Therapeutics |
| 11.2.1 | Drug Description |
| 11.2.2 | Other Development Activities |
| 11.2.3 | Clinical Trials Information |
| 11.2.4 | Safety and Efficacy |
| 11.2.5 | Analysts’ Views |
| 11.3 | Pozdeutinurad (AR882): Arthrosi Therapeutics |
| 11.4 | Lingdolinurad (ABP-671): Atom Therapeutics |
| To be continued in the report…. | |
| 12 | Chronic Gout Market: Seven Major Market Analysis |
| 12.1 | Key Findings |
| 12.2 | Key Chronic Gout Market Forecast Assumptions |
| 12.3 | Chronic Gout Market Outlook |
| 12.4 | Attribute Analysis |
| 12.5 | Total Market Size of Chronic Gout in the 7MM |
| 12.6 | Market Size of Chronic Gout by Therapies in the 7MM |
| 12.7 | The US Chronic Gout Market Size |
| 12.8 | Market Size of Chronic Gout in the EU4 and the UK |
| 12.9. | Japan Chronic Gout Market Size |
| 13 | Key Opinion Leaders’ Views on Chronic Gout |
| 14 | Chronic Gout Unmet Needs |
| 15 | Chronic Gout Market SWOT Analysis |
| 16 | Chronic Gout Market Access and Reimbursement |
| 16.1 | United States |
| 16.2 | EU4 and the UK |
| 16.3 | Japan |
| 17 | Bibliography |
| 18 | Abbreviations and Acronyms |
| 19 | Chronic Gout Market Report Methodology |
Related Reports
Chronic Gout Clinical Trial Analysis
Chronic Gout Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key chronic gout companies, including Teijin Pharma, Horizon Therapeutics, Sanwa Kagaku Kenkyusho Co. Ltd., Jiangsu Atom Bioscience and Pharmaceutical, Arthrosi Therapeutics, Teijin Limited, Selecta Biosciences, Inc., Shanton Pharma, among others.
Gout Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key gout companies, including Mylan N.V., MERCK & Co. Inc., Iroko Pharmaceutical LLC, Hikma Pharmaceuticals plc, Takeda Pharmaceutical Company Limited, Teijin Pharma Limited, Novartis AG, Horizon Therapeutics plc, Teva Pharmaceutical Industries Ltd., among others.
Gout Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key gout companies, including Arthrosi Therapeutics, Jiangsu Hengrui Medicine, LG Chem, InventisBio, Shanton Pharma, Dyve Biosciences, Protalix BioTherapeutics, Jiangsu Atom Bioscience and Pharmaceutical, among others.
Chronic Refractory Gout Market
Chronic Refractory Gout Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key chronic refractory gout companies, including Amgen, Cartesian Therapeutics, Swedish Orphan Biovitrum (Sobi), Arthrosi Therapeutics, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Pharmaceutical Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
Connect with us on LinkedIn|Facebook|Twitter
Contact Us
Shruti Thakur
info@delveinsight.com
+14699457679

Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
